June 13, 2025 — Beijing, China — In a research paper entitled "H2A.Z is essential for oocyte maturation and fertility in female mouse " published in Nature Structural & Molecular Biology, a collaborative research team led by Wei Xie’s team from Tsinghua University, in partnership with Yu Zhang’s group from Fudan University and Yunlong Xiang’s group from Chongqing Medical University, has revealed a pivotal role of the histone variant H2A.Z in regulating oocyte maturation and fertility in female mice.
Oocyte maturation is essential for both gametogenesis and early development, during which large amounts of transcripts are produced. However, how transcription is regulated in oocytes remains largely unclear due to limited research materials. The transcription regulatory circuitry and the cis-regulatory elements (CREs) such as promoters and enhancers, are still poorly defined in mammalian oocytes. Of note, global transcription in oocytes occurs in the absence of DNA replication. Histone variants, which can be incorporated into chromatin in a DNA replication-independent manner (unlike that of canonical histones) are naturally suited for such regulation. Currently, it is unclear whether CREs in oocytes undergo preferential histone variant exchanges, and if so, how they are regulated remains to be investigated.
In this study, the researchers demonstrated that H2A.Z is predominantly localized at active enhancers and promoters, but not at poised promoters, in fully grown oocytes (FGOs). Ablation of H2A.Z in oocytes leads to severe defects including meiotic arrest, impaired chromatin condensation, and ultimately female infertility. Mechanistically, H2A.Z facilitates CRE activity as H2A.Z mutation leads to reduced active chromatin features associated with transcription, such as H3K27ac and H3K4me3, which is accompanied by transcriptional dysregulation and impaired oocyte developmental competence. In sum, this work identifies H2A.Z as a key chromatin regulator in oocytes, highlighting its role in maintaining enhancer and promoter function during oocyte maturation. These findings emphasize the broader significance of histone variants in regulating gene expression programs that control gametogenesis and the maternal-to-zygotic transition.
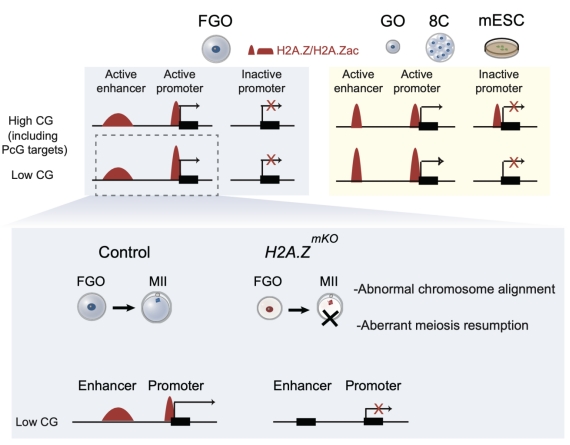
A model illustrating the functions of H2A.Z in mouse oocytes
The study is jointly led by Prof. Wei Xie (Tsinghua University), Associate Prof. Yu Zhang (Fudan University), and Prof. Yunlong Xiang (Chongqing Medical University). Assistant Professor Qianhua Xu and Dr. Chunyi Huang from Tsinghua University are co-first authors. This study was conducted in collaboration with Prof. Guohong Li’s laboratory at the Institute of Biophysics, Chinese Academy of Science. Additional support was provided by the Animal Center and the Bioinformatics Core Facility at Tsinghua University. The project was funded by the National Natural Science Foundation of China, the Key R&D Program of the Ministry of Science and Technology, the Tsinghua-Peking Center for Life Science and the New Cornerstone program.
Paper link: https://www.nature.com/articles/s41594-025-01580-y
